Describes Pure Water Relative to Plasma
Terms in this set 5 What do you need to make pure water. The fusion or budding of transport vesicles at the plasma membrane either adds or removes proteins and phospholipids thus changing the surface area.
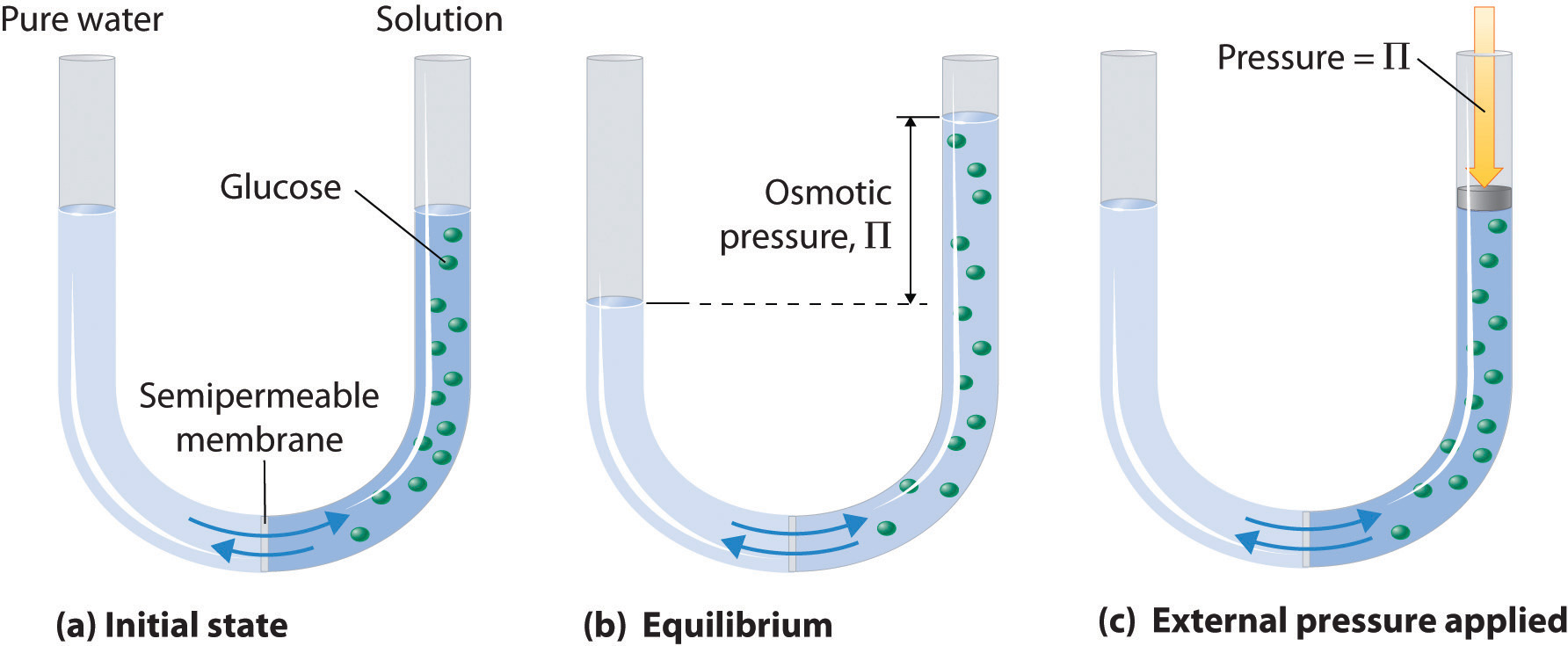
3 6 Osmotic Pressure Chemistry Libretexts
Describes 10 dextrose solution relative to plasma if 5 dextrose solution is isotonic to plasma.

. Water moves across plasma membranes by a specific type of diffusion called osmosis. Thus if the membrane is permeable to water and not solutes osmotic pressure is proportional to the difference in solute concentration across the membrane the proportionality factor is RT. Out of the extracellular fluid volume 75 or 105L of the volume is present in the interstitial space and 25 of that water is in the plasma which is equivalent to 35L.
So plasma in water or other liquids is not only possible but now used in hospitals. Nephrology Physeo Guide. Generally speaking by the time a gas is hot enough to be seen its a plasma.
The reason this is possible is because many real plasma are in non equilibrium. Figure 2623 Aquaporins. This means that temperature is not defined.
The ECFV is comprised of two spaces. A common example of a chemical substance is pure water. Cholesterol is also present between the phospholipids which contributes to the fluidity of the membrane.
Here are 10 examples of forms of plasma. The fundamental structure of the membrane is the phospholipid bilayer which forms a stable barrier between two aqueous compartments. Definitions The fourth state of matter.
There are various proteins embedded within the membrane that have a variety of functions. That is the gas is so hot and the atoms are slamming around so hard that some of the electrons are given enough energy to temporarily escape their host atoms. Normally we specify the following quantities when we describe a plasma.
Water potential is the potential energy of water in a system compared to pure water when both temperature and pressure are kept the same. Water Potential Definition. It forms is an bubble in the liquid.
That is water moves through channel proteins called aquaporins from higher water concentration to lower water concentration. Shrinking of red blood cells. It is a plasma is liquid.
YOU MIGHT ALSO LIKE. We divide plasmas into two general types as follows. Natural water contains dissolved salts you need to remove these.
It is measured in kilopascals kPa and is represented by the. Plasma is called the fourth state of matter after solid liquid and gas. Solution that is more concentrated than the concentration inside the cell.
Stars including the Sun the tail of a comet. Like all other cellular membranes the plasma membrane consists of both lipids and proteins. Solution that causes crenation of a red blood cell.
Blood plasma by the way is something completely different. The four main states of matter are solids liquids gases and plasma. A gas lacks either a defined shape or volume.
P RT ΔC where ΔC is the difference in solute concentration between the two solutions. In the case of the plasma membrane these compartments are the inside and the outside of the cell. Describes pure water relative to plasma.
In the body water moves by osmosis from plasma to the IF and the reverse and from the IF to the ICF and the reverse. Bursting of red blood cells. The plasma membrane is an extremely pliable structure composed of 2 layers of back-to-back phospholipids a bilayer.
It is the liquid portion of blood. An organism with a cell wall would most likely be unable to take in materials through ____. It always has the same properties and the same ratio of hydrogen to oxygen whether it is isolated from a river or made in a laboratory.
The concentration gradient of water across a membrane is inversely proportional to the concentration of solutes. Blood is 60 plasma and 40 other stuff like red blood cells white blood cells platelets and etc. A single phospholipid molecule has a phosphate.
Describes a 10 dextrose solution relative to plasma if a 5 dextrose solution is isotonic to plasma. Boiling water to make steam and then steam condenses. So plasma is what contains the water it is 92 water as stated and is 60 of bloods tota.
We first describe what a plasma is how it behaves under the influence of electric and magnetic fields and how it is characterized. Plasma is typically an electrically quasineutral medium of unbound positive and negative. A solid has a definite shape and volume.
Describes 10 dextrose solution relative to plasma is a 5 dextrose solution is isotonic to plasma. Composition electron and ion temperatures and electron and ion densities. Water H2 O is a polar inorganic compound that is at room temperature a tasteless and odorless liquid nearly colorless with a hint of blueThis simplest hydrogen chalcogenide is by far the most studied chemical compound and is described as the universal solvent for its ability to dissolve many substances.
Solution that causes crenation of a red blood cell. The binding of ADH to receptors on the cells of the collecting tubule results in aquaporins being inserted into the plasma membrane shown in the lower cell. These two changes the surface area of the plasma membrane.
The excited low-pressure gas inside neon signs and fluorescent lights. For example if you are sweating you will lose water through your skin. Indeed water as found in nature.
Too expensive need a lot of energy to distil all the. The interstitial fluid volume ISFV and the plasma volume PV. Under exceptional conditions other states of matter also exist.
It is a state of matter in which an ionized substance becomes highly electrically conductive to the point that long-range electric and magnetic fields dominate its behaviour. The big difference between regular gas and plasma is that in a plasma a fair fraction of the atoms are ionized. A liquid has a definite volume but takes the shape of its container.
Other chemical substances commonly encountered in pure form are diamond carbon gold table salt sodium chloride and refined sugar sucrose. Proteins embedded within the phospholipid bilayer. It can also be described as a measure of how freely water molecules can move in a particular environment or system.
Describes pure water relative to plasma. One-third of the total body water is the ECFV which is equivalent to 14L. This allows it to be the solvent of life.
Bursting of red blood cells. In the body water moves constantly into and out of fluid compartments as conditions change in different parts of the body. This dramatically increases the flow of water.
It is 92 percent water and constitutes 55 percent of blood volume according to the American Red. Plasma is made up of 92 water and 8 other stuff like minerals Sodium and Potassium and organic stuff like hormones amino acids and etc. Consists of two phases interphase.
Describes pure water relative to plasma. Red Blood Cell Membrane Permeability 18 Terms.

Pure Water Absorption Spectra Measured As A Function Of Temperature Download Scientific Diagram

What Would Happen To A Cell Placed In Pure Water Quora

Pure Water Absorption Spectra Measured As A Function Of Temperature Download Scientific Diagram

4 13 Tonicity Water Movement Pure Products Science Education
No comments for "Describes Pure Water Relative to Plasma"
Post a Comment